
- #BLANK PRINTABLE PERIODIC TABLE OF ELEMENTS HOW TO#
- #BLANK PRINTABLE PERIODIC TABLE OF ELEMENTS SERIES#
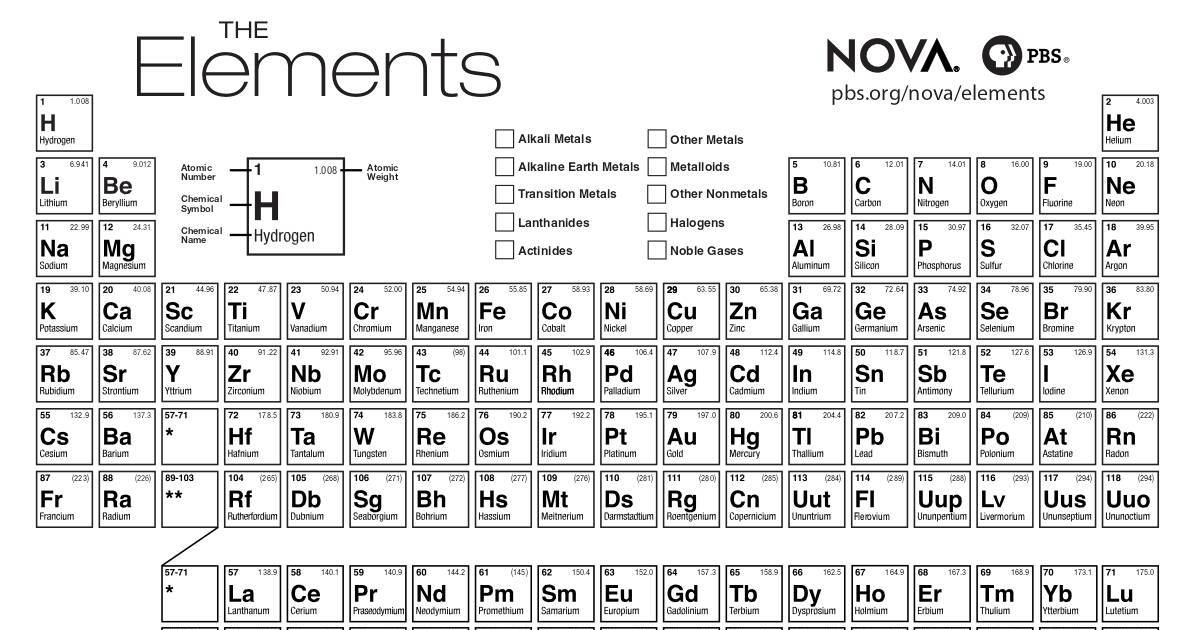
This is at the top of the cell, in the center or to the left. However, most include two pieces of information.
f-block- usually placed below the table, the lanthanides and actinidesĮach printable periodic table can include different information in a cell. s-block- the alkali metals, the alkaline earth metals, helium and hydrogen. There are also four blocks, each containing different groups. Elements with similar chemical characteristics are given a group name and colored the same color, in some tables. Rows are called periods, and columns are called groups. #BLANK PRINTABLE PERIODIC TABLE OF ELEMENTS HOW TO#
How to use a Printable Periodic Table of Elements Little is known about some of these elements because they are unstable and decay rapidly. They completed the seventh row of the table.
:max_bytes(150000):strip_icc()/PeriodicTableValence-58b5d8f95f9b586046df59fb.jpg)
The most recent of these were officially recognized in 2015. Since the 1940s, scientists have discovered the transuranic elements, numbers 104-118. Fellow scientists discredited his suggestion at first but later research proved he was correct.
#BLANK PRINTABLE PERIODIC TABLE OF ELEMENTS SERIES#
In 1945, he published a paper that said the actinide series of elements should be added to the periodic table as the second row of the f-block, below the lathanide elements. Seaborg began speculating that additions needed to be made to the periodic table. Since then, the atomic number of an element has been based on the number of protons it contains.ĭuring his work on the Manhattan Project, Glenn T. It was during the middle part of the 20th century that scientists discovered protons. In 1923, Horace Groves Deming published the 18 column periodic table that is common today. Mendeleev later adjusted his table to correct the position of some elements. Two, it would allow future scientists to adjust the position of elements within the table without damaging its integrity. One, it allowed for the addition of new elements and groups of elements he did not predict. Mendeleev’s addition of dashes to his table was important for two reasons.
Mendeleev said an elements’ properties could be predicted by using its mass and its position in the periodic table. He moved some elements so they were beside elements with similar characteristics regardless of their mass. This helped him realize that some elements were in the wrong place when they were ordered by mass. He also realized that elements with similar atomic masses had similar chemical properties. Medeleev arranged his table by atomic mass. Using a dash, Mendeleev left spaces in his table where he thought those elements should be placed. He speculated about the existence of undiscovered elements. His table not only documented known elements, it allowed space for the discovery of new ones. Mendeleev is credited with the development of the modern periodic table because his work was the most innovative and adaptable. In the 1860s, three men independently published periodic tables: Dimitri Ivanovich Mendeleev, Lothar Meyer, William Odling. Newlands work was not appreciated at the time, but today, he is credited with the first use of the term periodic in chemistry. He also paired elements with masses that differed by a factor of eight. His number was based on an element’s mass. He was the first scientist to assign elements an atomic number. John Newlands published works in the early 1860s that divided 62 known elements into groups of eight. He divided his elements into two groups, metals and nonmetals. In it, he identified several new elements, including hydrogen, nitrogen and oxygen. It was the first textbook devoted to chemistry. In 1789, Antoine-Laurent de Lavoisier wrote the Elementary Treatise of Chemistry. Scientists used this definition until subatomic particles were discovered. He defined an element as something that cannot be broken into smaller parts by a chemical reaction. Robert Boyle developed the definition of an element around 1650 that was used for 300 years. Below are some of the people whose work contributed to the formation of the periodic table. Scientists continue to find elements and learn about them today. 
This led to the discovery of new elements. At this time, people began conducting chemical experiments. The work that led to the periodic table began during the Age of Enlightenment. The study of the elements goes back to ancient times.



:max_bytes(150000):strip_icc()/PeriodicTableValence-58b5d8f95f9b586046df59fb.jpg)



 0 kommentar(er)
0 kommentar(er)
